
PharmaShots Weekly Snapshots (December 23rd – December 27th, 2024)
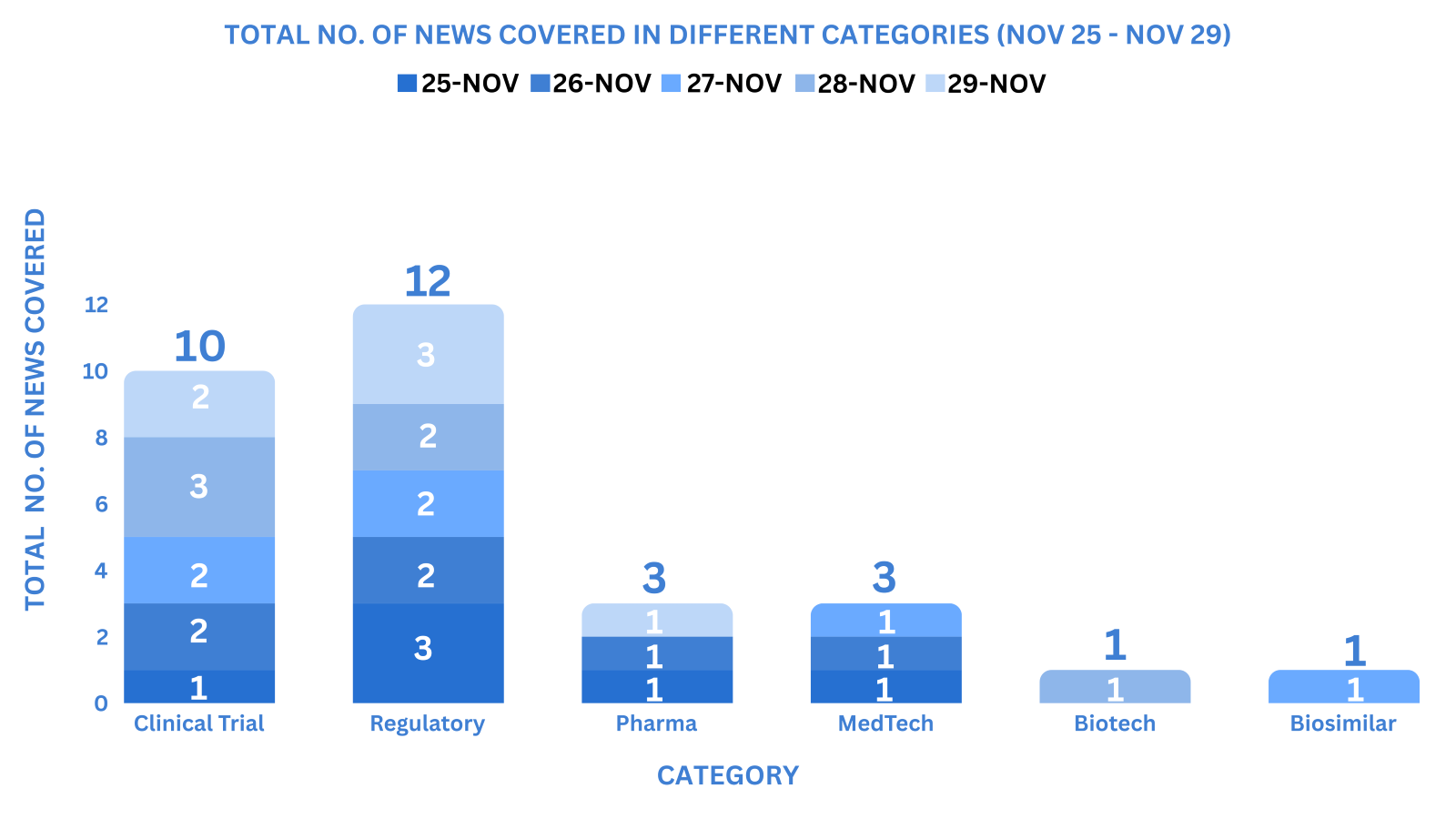
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, Biosimilars & Biotech. Check out our full report below:

Merck Reports Topline Data from P-III Studies of Doravirine/Islatravir (DOR/ISL) Regimen to Treat Virologically Suppressed HIV-1 Infection
Read More: Merck
Arrowhead Pharmaceuticals Commences P-I/IIa Trial of ARO-INHBE to Treat Obesity
Read More: Arrowhead Pharmaceuticals
Apollomics Reports Top-line Data from P-III Bridging Study of Uproleselan in Chinese Patients with R/R Acute Myeloid Leukemia (AML)
Read More: Apollomics
BMS Reports Topline Data from Two Pivotal P-III Studies of Sotyktu (Deucravacitinib) for Treating Psoriatic Arthritis (PsA)
Read More: BMS
Ractigen Therapeutics Reports the First Patient Dosing with RAG-17 in P-I Study to Treat Amyotrophic Lateral Sclerosis (ALS)
Read More: Ractigen Therapeutics
Galimedix Therapeutics Commences Pivotal P-II Trial of GAL-101 Eye Drops for Treating Dry AMD
Read More: Galimedix Therapeutics
Zura Bio Reports Global P-II (TibuSURE) Trial of Tibulizumab to Treat Adults with Systemic Sclerosis
Read More: Zura Bio
Immunocore Reports the First Patient Dosing with IMC-P115C in P-I Study to Treat Tumors Expressing PRAME
Read More: Immunocore
Assembly Biosciences Reveals Interim Data from P-Ib Study of ABI-4334 to Treat Chronic Hepatitis B
Read More: Assembly Biosciences
Sensorion Reports the Completion of Patient Recruitment in P-I/II (Audiogene) Gene Therapy Clinical Trial
Read More: Sensorion

Eli Lilly Reports the US FDA’s Approval of Zepbound (Tirzepatide) to Treat Moderate-to-Severe Obstructive Sleep Apnea in Obese Adults
Read More: Eli Lilly
Pfizer’s Braftovi (Encorafenib) Secures the US FDA’s Accelerated Approval as a 1L Treatment of BRAF V600E-Mutant Metastatic Colorectal Cancer
Read More: Pfizer
AstraZeneca’s Tagrisso (Osimertinib) Secures the EC’s Approval to Treat Unresectable EGFR-Mutated Lung Cancer
Read More: AstraZeneca
BMS Reports the EC’s Approval of Opdivo (Nivolumab) Plus Yervoy (Ipilimumab) as a 1L Treatment of MSI-H/dMMR Metastatic Colorectal Cancer (mCRC)
Read More: BMS
Rhythm Pharmaceuticals Reports the US FDA’s Approval of Imcivree (Setmelanotide) for Patients as Young as 2 Years Old
Read More: Rhythm Pharmaceuticals
Sumitomo Pharma’s Gemtesa (Vibegron) Secures the US FDA’s Approval to Treat Overactive Bladder (OAB) Symptoms in Men
Read More: Sumitomo Pharma
Nuvation Bio Reports the US FDA’s NDA Acceptance of Taletrectinib with Priority Review to Treat Advanced ROS1+ NSCLC
Read More: Nuvation Bio
Anavex Life Sciences Reports the EMA’s MAA Acceptance of Blarcamesine for Treating Alzheimer’s Disease
Read More: Anavex Life Sciences
Tonix Pharmaceuticals Reports the US FDA’s NDA Acceptance of TNX-102 SL for Treating Fibromyalgia
Read More: Tonix Pharmaceuticals
Takeda’s Hyqvia 10% SC Injection Receives the Japanese Approval to Treat Agammaglobulinemia or Hypogammaglobulinemia
Read More: Takeda
Daiichi Sankyo’s Datroway Receives the MHLW’s Approval for Unresectable or Recurrent HR+/HER2- Breast Cancer
Read More: Daiichi Sankyo
Novo Nordisk’s Alhemo Receives the US FDA’s Approval as a Prophylactic Treatment of Hemophilia A or B with Inhibitors
Read More: Novo Nordisk

Astellas and Sangamo Therapeutics Partner for Administering Genomic Therapies Targeting Neurological Disorders
Read More: Astellas and Sangamo Therapeutics
RAPT Therapeutics and Jemincare Pharmaceutical Sign an Exclusive License Agreement to Develop JYB1904 (RPT904)
Read More: RAPT Therapeutics and Jemincare Pharmaceutical
AusperBio Raises $73M in Series B Financing to Develop AHB-137 for Chronic Hepatitis B
Read More: AusperBio
 Mainz Biomed Partners with Quest Diagnostics to Commercialize its Colorectal Cancer Screening Test
Mainz Biomed Partners with Quest Diagnostics to Commercialize its Colorectal Cancer Screening Test
Read More: Mainz Biomed and Quest Diagnostics
Signati Medical Reports the IDE Trial Completion of Separo Vessel Sealing System for Vasectomy Procedures
Read More: Signati Medical
MedMira’s Multiplo(R) Rapid (TP/HIV) Test Secures the Health Canada’s Approval for Syphilis and HIV
Read More: MedMira
 Bio-Thera Solutions and Tabuk Pharmaceuticals Join Forces to Commercialize BAT2206 (Biosimilar, Stelara) in Saudi Arabia
Bio-Thera Solutions and Tabuk Pharmaceuticals Join Forces to Commercialize BAT2206 (Biosimilar, Stelara) in Saudi Arabia
Read More: Bio-Thera Solutions and Tabuk Pharmaceuticals

Axcelead DDP Collaborates with Astellas to Discover Candidates for Targeted Protein Degraders
Read More: Axcelead DDP and Astellas
Related Post: PharmaShots Weekly Snapshots (December 16 – December 20, 2024)
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














